Your Cart is Empty
Local Shipping within US, Canada, EU and Australia
Menu

Local Shipping within US, Canada, EU and Australia
Mixing dry aquarium fertilisers
February 04, 2025 7 min read

What are dry fertilisers?
Dry aquarium fertilisers are fertilisers that are supplied as pure chemical salts. These salts dissolve in water to provide plants with the nutrients they need. They are extremely concentrated because they come in pure chemical salt form. Most commercial fertilisers are made from the same basic salts - except that they are pre-mixed with water and come with dosage instructions. However, hobbyists can buy the dry salts directly from dry powder suppliers.
For example, you can buy a bottle of XXX brand 'potassium' liquid fertiliser to add potassium to your planted tank. This bottle can instead be replaced by dosing the dry salt K2SO4/potassium sulphate at a fraction of the cost. In fact, when we subject the contents of commercial fertilisers to laboratory analysis, most of them are made up of these similarly available salts.
The difficulty in mixing your own is twofold - firstly, handling the different salts required to cover all the nutrients. Secondly, the measurement and calculation involved in determining the dosage rates for a particular tank. However, most salts are non-hazardous and easy to handle, and with online calculators available, it is easy to come up with your own dosing solutions.
What you need
First you need the dry salts. The essential nutrients, as mentioned in previous sections, are NPK, Fe + Traces, and perhaps Ca/Mg if your water is deficient in these. For each nutrient there is a corresponding salt that contains it. Some salts provide more than 1 type of nutrient.
| COMMON SALTS | |
| Potassium | Potassium sulfate (K2SO4), Potassium chloride (KCl), Potassium nitrate (KNO3), Monopotassium phosphate (KH2PO4). |
| Nitrogen | Potassium nitrate (KNO3), Calcium nitrate (Ca(NO3)2.4H2O) |
| Phosphorus | Monopotassium phosphate (KH2PO4), Dipotassium phosphate (K2HPO4) |
| Magnesium | Magnesium sulfate (MgSO4) |
| Iron | Iron is a reactive element and Iron fertiliser should come in chelated format - this means that the iron atom is bound to a chelating agent, which keeps it in Fe2+ oxidation state which is preferable for plant uptake, rather than being oxidised into (Fe3+) state. Many trace element mixes such as Plantex CSM + B already contain iron and you do not require additional iron if using those mixes. |
| Traces | These come under common packages that contain all 5 essential trace elements: Plantex CSM + B, MicroMix plus, Miller's Microplex, Plant-prod Micro mix, TNC Trace |


EQUIPMENT
MIXING BOTTLES
One way of adding nutrients to the tank is to dose dry salts directly into the tank water and allow them to dissolve in the tank environment. However, many aquarists prefer to mix their own dosing solutions - which may contain a number of different salts. This eliminates the need to measure each salt when dosing. Mixed solutions also allow greater precision when dosing in small tanks. For example, the amount of phosphate salt required for a small tank may be so small that it is difficult to measure - diluting a larger amount of dry salt into a solution and dosing the solution gives greater accuracy.
WEIGHING SCALE / MEASURING DEVICE
A scale accurate to at least 1 gram; many kitchen scales will do. Seachem sells a digital measuring spoon accurate to 0.1 grams for about $20. So if you're going to spend money on a scale anyway, it's worth considering. If you're on a super tight budget where cost minimisation is a priority, a set of accurate measuring spoons will also work - there will be some margin of error, but planted tanks work over a wide enough range that it won't matter most of the time. Plastic spoons don't rust after interaction with salts, unlike metal ones.


How to measure Dry Salts
Firstly, target levels for nutrients in the tank are given in ppm (parts per million). So if someone says they have 1 ppm of nitrates in the tank, this means that for the given mass of water in the tank, divide by one million and that is the mass of nitrates available in the tank. So 1 ppm of nitrates in a tank containing 1 million kg of water means that the tank contains 1 kg of nitrates (1 out of 1 000 000).
The target levels for each nutrient are different and this is covered on the nutrient dosing approaches page. For example, EI recommends dosing up to 30ppm of potassium per week.
How does one measure a dose for 30ppm of potassium for a 100 litre tank size?
We can easily make use of online nutrient dosing calculators such as this one
This approach demonstrates dosing the dry powder directly into the tank.
1. Enter your tank size in the column

2. Click on "DIY"

3. Next select your compound. We will use K2SO4 (potassium sulfate) in this demo. Then click on "dry dosing". This means that we will be adding the dry salt directly into our tank.
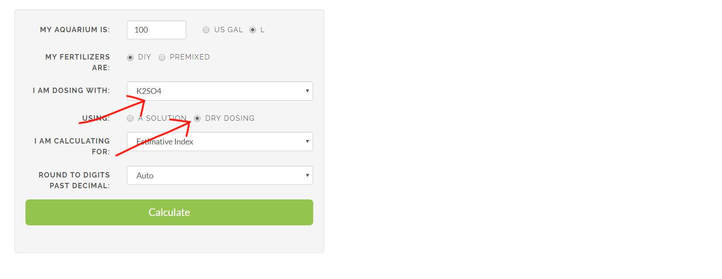
4. Next choose "Dose to reach a target" under the column "I am calculating for"
5. Since we have a target of 30ppm of K, enter 30ppm in the "My target is" box.

5. Press the calculate button. The results will come out on the right side. In this scenario, to get 30ppm of potassium into a tank of 100 litres, we would need to add 6.69grams of the salt K2SO4 into the aquarium.

Alternatively, we can use the calculator to estimate the result of a given dose. For example, if we only have teaspoons and are wondering how much potassium 1 teaspoon of K2SO4 would add to the tank of 100 litres.
Click on the 'I am calculating for' column and select 'Result of my dose'.
Then enter the dose of K2SO4 you would add to the tank. In this demo we have chosen 1 teaspoon.
The results will then appear on the right hand side: 1 teaspoon of K2SO4 to our 100 litre tank will add 28.72ppm of potassium to our tank.

Creating a dosing solution
The next approach shows how to dose fertiliser by creating a dosing solution. I.e. making your own liquid fertiliser.
Steps 1 and 2 are the same as above. However, instead of clicking on Dry dosing, we click on 'A solution' under the 'Use' column.
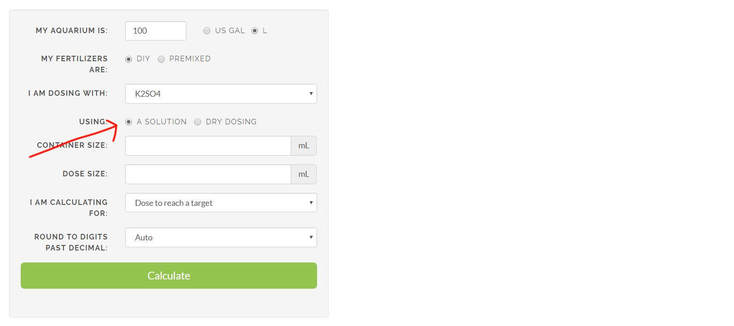
3. Enter your container size. This refers to the volume of the fertilizer solution that you are going to create. Here we choose to use a 500 ml bottle.

4. Dose size refers to the dose of liquid fertilizer solution that you are going to add into your aquarium each time you dose. This may vary depending on the size of your aquarium, and frequency of dosage. In this case we entered 20ml as our dosage size. "My target is" column refers to the amount of ppm you want added to the tank when you do 1 dose, here we entered 10ppm. This means that we expect our 20ml solution to add 10ppm of potassium to our tank.

5. Click on the calculate button and the site will tell you that you need to add 55.71grams of K2SO4 to your 500ml bottle of solution. Each 20ml dose would then add 10ppm of potassium to the tank. If you have selected a target dose that is too high, or dosage size that is too small, the site will give you the warning that one cannot dissolve so much of a particular salt in the volume of water. In this case we need to tweak the dosage size, or target ppm. Alternatively, we can choose chemicals/salts that are more soluble.

Similar to dry dosing, one can use the "Result of my dose" option under "I am calculating for" column to get an estimate of how much ppm our dose adds to a tank if we dissolved the indicated amount of salt in our solution bottle. Here the estimation is that if we dissolve 50 grams of K2SO4 in 500ml of water, a 10ml dose of the solution into a 100 litre tank would give 4.49ppm of K. Again, if you enter too large an amount of salt to be dissolved in the container size, a warning message would appear.

WALKTHROUGH EXAMPLE
Here we go through making a macros (NPK) and micros (Fe/Traces) dosing solution. The reason to have 2 bottles is that phosphates can bind to iron easily, creating an insoluble precipitate, so keeping them in separate bottles prevents that. We would dose each bottle on Mon/wed/fri. While phosphates and iron would bind in a concentrated solution - the dilution in the tank environment prevents this from happening, so dosing on the same day is fine.
Firstly, let us define our target variables.
1. We want to dose only 3 times a week, on Monday/Wed/Fri.
2. We need to define our target levels:
Each dose of our macro solution will add:
- 4ppm Potassium (12ppm per week)
- 3ppm Nitrates (9ppm per week)
- 1ppm Phosphates (3ppm per week)
- 1ppm Magnesium (3ppm per week)
Each dose of our micros solution will add:
- 0.1ppm Iron/traces (iron used as a proxy) (0.3ppm per week)
In addition, we would be using 500ml bottles for each solution.
The salts we would use:
For macro solution:
- KCl - provides Potassium
- KNO3 - provides both Potassium & Nitrates
- KH2PO4 - provides both Potassium & Phosphates
- MgSO4 - provides Magnesium
For micros solution:
- Plantex CSM + B - provides iron/traces
So we enter the nutrient dosing calculator website.
1. Enter aquarium size (for example, 46gallons)
2. Choose "DIY"
3. we'll start with calculating for KNO3, which provides both potassium and nitrates
4. Choose "A solution"
5. Our container size is 500ml
6. Each dose we would want to add to the tank is 10ml - we estimate that this is a reasonable dosage size for this tank
7. Choose "Dose to reach a target"
8. Our target for NO3 is 3ppm as from above.
9. Press calculate.

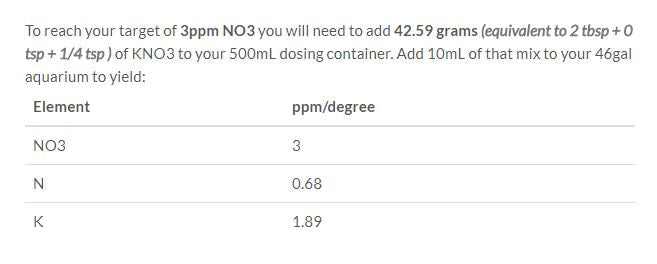
On the right hand side, the calculator tells us that we have to add about 43 grams of KNO3 to our dosing solution to get 3ppm per dose for nitrates.
We repeat the steps for KH2PO4, MgSO4 by changing the chemical selected at step 3. & dosing target at step 8.


Lastly we would calculate for KCl.
As KNO3 and KH2PO4 both add potassium to the tank, we need take that into account.
Our target is 4ppm of potassium per dose.
KNO3 and KH2PO4 already add (1.9ppm + 0.4ppm of potassium), so we just need to add 1.7ppm more K. (4ppm - 1.9ppm - 0.4ppm)
We enter 1.7ppm as our target at step 8. after choosing KCl at step 3.
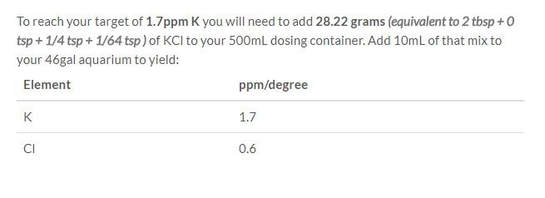
Summarizing the above:
For our Macro dosing solution of 500ml:
500ml water
28.2 grams of KCl
42.6 grams of KNO3
12.5 grams of KH2PO4
88.3 grams of MgSO4
(rounding off to the nearest gram is fine)
10ml per dose of the above will add the below to our 46 gallon tank:
4ppm Potassium
3ppm Nitrates
1ppm Phosphates
1ppm Magnesium
For our Micros dosing solution, it's a single step process
500ml water
13.3 grams of Plantex CSM + B
10ml per dose of the above will add the below to our 46 gallon tank:
0.1ppm Fe
Traces as proportionate ratio to Fe above.

Grow anything, defeat algae, create amazing aquascapes

















